 1
1 1
1
By Ahmed Aboulenein
WASHINGTON (Reuters) – The U.S. government will soon begin hiring experts and collecting the data needed to launch direct negotiations over prescription drug prices for older and disabled people, a top Biden administration official told Reuters.
President Joe Biden last week signed into law the Inflation Reduction Act, introducing new policies to tackle climate change, taxes and the rising cost of medicines.
The Act will for the first time allow the federal Medicare health plan for people age 65 or older and the disabled to negotiate prices on up to 20 drugs a year. It also sets limits on drug price increases for Medicare and caps out-of-pocket costs for those enrolled in the program.
The move represents a rare legislative defeat for the powerful pharmaceutical industry and sets a precedent for curbing drug prices in the world’s most lucrative market for medicines.
“We definitely are looking to increase our expertise,” Chiquita Brooks-LaSure, administrator of the Centers for Medicare and Medicaid Services, said in an interview. “This has been a long time coming, for the federal government and Medicare to have this authority.”
Brooks-LaSure said she plans to create a new team tasked with negotiating drug prices within the Center for Medicare. CMS says it expects to start hiring for over 100 positions beginning this fall.
“We are really looking for clinical expertise … as well as people who have experience negotiating,” said Brooks-LaSure, whose agency has regulatory oversight of nearly all healthcare providers in the United States and control of federal health programs covering 170 million people, including 64 million enrolled in Medicare.
“One of the first things is for us to choose which drugs to negotiate. We have 10, and that is something that we’ll be announcing next fall, so around a year from now,” she said.
CMS will need to collect data from pharmaceutical manufacturers, health insurers and pharmacy benefit managers (PBMs) to identify those first 10 drugs that will be subject to negotiations, a list that will rise to 20 by 2029, she said.
DATA COLLECTION
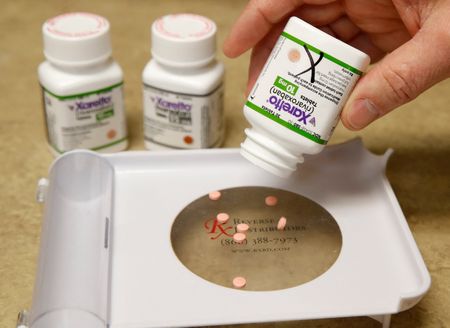
The government will choose from 50 “high spend” drugs based on Medicare utilization and cost that have only one supplier. Healthcare analysts have said that list could include Bristol-Myers Squibb Co’s top-selling cancer drug Revlimid, AbbVie Inc’s rheumatoid arthritis drug Humira, and blood thinner pill Xarelto which is sold by Johnson & Johnson in the United States.
But full drug price information is often obscured in the U.S. market. Drugmakers, who fought tooth and nail to prevent the new law from passing, say they often provide substantial rebates and discounts to health plans that are not passed on to consumers. Health plans argue that drugmakers are responsible for setting, and raising, the price of their medicines.
The pharmaceutical industry’s powerful trade association, Pharmaceutical Research and Manufacturers of America (PhRMA), declined to comment on how its members might contribute information to CMS or if any of them had been approached.
Officials representing the largest three PBMs – who help negotiate drug prices for health plans – did not respond to requests for comment.
AHIP, the health insurance industry’s largest trade group, and its members “stand ready to assist” the government “on this or other implementation issues for new policies,” spokesperson David Allen said.
MOVING FAST
Health policy experts say CMS needs to hire from the outside because it does not currently have the capacity to negotiate drug prices. To have an impact, the agency will need to move quickly, because the law requires it to start negotiating in 2023, with the new prices taking effect in 2026.
“The idea of negotiating drug prices has been talked about for quite a while, but this legislation came together very fast. So the federal government is going to have to move quickly to put in place an infrastructure here,” said Larry Levitt, executive vice president for health policy at the Kaiser Family Foundation.
Brooks-LaSure said she would cast a wide net and is looking to hire experienced public and private-sector negotiators as well as drug-pricing experts.
“We are absolutely looking to hire from within our friends in the administration as well people outside, and folks will start to see postings on our CMS website,” she said. “So whether it’s the VA, whether it’s from the outside world, from the commercial market, welcome.”
While the Department of Veteran Affairs (VA), which covers healthcare for more than 9 million people, negotiates drug prices, it does so on a much smaller scale than Medicare would be expected to.
Brooks-LaSure acknowledged the difference and said, “there will be some new uniquely CMS approaches” to negotiations.
CMS should also look to groups like the Institute for Clinical and Economic Review (ICER), an influential Boston-based nonprofit that researches drug prices, said Andy Slavitt, a former CMS chief under President Barack Obama.
“These are challenging negotiations where people are forced to eat the dog food they don’t want to eat,” Slavitt said.
(Reporting by Ahmed Aboulenein in Washington; Editing by Michele Gershberg and Matthew Lewis)